PCOS and PCOD: Learn the Symptoms, Differences, and Treatment Options
 21 November,2024
Read More
21 November,2024
Read More
Enquire now in case of any assistance needed
Cellular immunotherapies are the new age cancer treatment. With advances in medical technologies, researchers are constantly improving the scenario of cancer management. CAR T-cell therapy is the latest addition to it. The US FDA approved CAR T-cell therapy for cancer patients in 2017. India's first CAR T-cell therapy was administered on June 4, 2021, at Tata Memorial Center, Mumbai.
If you are also fascinated by the news of this innovative cancer treatment and want to know more about it, you have landed at the right place. This blog aims to provide all the relevant information about CAR T-cell therapy, including the procedure, cost, and related side effects.
The primary treatments for most cancers are surgery, chemotherapy, and radiotherapy. Since the 2000s, cancer treatment has shifted from traditional approaches to more personalized ones using targeted and immunotherapy.
CAR T-cell therapy is an immunotherapy that strengthens the body's immune system to spot and attack cancer cells. T-cells and B-cells are the main lymphocytes that fight off invaders in our body. T-cells do it by identifying specific proteins (antigens) present on the surface of foreign cells. T-cells have receptors that bind to these antigens and destroy them.
CAR T-cell therapy involves modifying and reengineering T cells in specially designed labs to enhance their capacity to find and destroy cancer cells. It has the advantage of staying active in the body for a prolonged period. Hence, it is a one-time therapy that reduces cancer recurrence chances.
CAR T-cell therapy focuses primarily on treating leukemia and lymphomas. It benefits people with previous treatment failures, including bone marrow or stem cell transplants. Patients experiencing recurrence of leukemia and lymphoma are also candidates for CAR T-cell therapy.
The following types of cancers have shown promising results when treated with CAR T-cell therapy –
Multiple myeloma is a recent addition to the approved diseases for CAR T-cell therapy.
CAR T-cell therapy is not advisable for everyone. Only specific tumors can be treated using this treatment. Solid tumors do not have a single protein that the CAR T-cells can target. Hence, CAR T-cell therapy is effective against solid tumors of the lungs, breasts, etc. Other contraindications for the therapy may include:
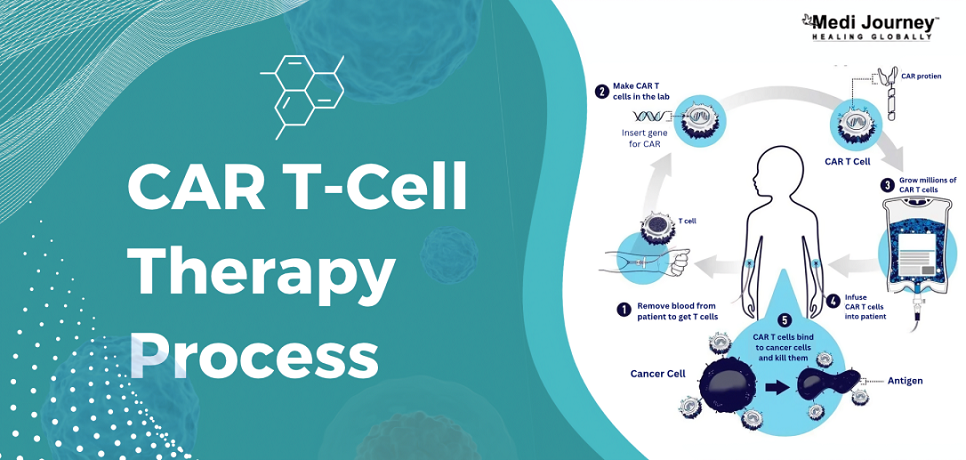
CAR T-cell therapy is one of the most promising cancer immunotherapy. It leverages the patient's immune system to target and eliminate cancer cells. The process involves the following steps –
Chemical Antigen Receptor (CAR) T-cell therapy is not suitable for everyone. You should consult an experienced cancer specialist to better understand whether you are an appropriate candidate for it. They will also educate you about a few potential complications the therapy may possess.
The most common complication of CAR T-cell therapy is CRS - cytokine release syndrome. CAR T-cells release chemicals called cytokines, which cause a reaction from the immune system. Signs and symptoms of CRS include:
CAR T-cell therapy may also cause harmful effects on the central nervous system. The signs and symptoms may include:
Other serious side effects that you may experience and require medical attention include:
You must have heard about specific treatments that cost several crores. CAR T-cell therapy used to be one of them, but not anymore. While the cost of treatment in the USA and UK ranges from USD 3,00,000 to 5,00,000, it is much more affordable in India.
India has developed its own indigenous CAR T-cell therapy, NexCAR19. In October 2023, the CDSCO - Central Drugs Standard Control Organization approved its use for several blood cancers. NexCAR19 costs around INR 35-40 lakhs or USD 40,000 to 50,000, which is 80-90% less than what other countries offer.
CAR T-cell therapy has become a boom in cancer treatment. It is currently available to treat blood cancers, but with ongoing research, the future is promising for other cancers. CAR T-cell therapy most benefits people with cancer recurrences and those who cannot be managed with other treatment modalities. However, it is essential to remember that a few side effects might be related to the therapy. It is best to weigh the risks and benefits with a skilled oncologist who can help you decide better.
Doctor of Pharmacy
Dr. Deepanshu Siwach is a skilled clinical pharmacist with a Doctor of Pharmacy degree. He has 4+ years of experience and has worked with thousands of patients. He has been associated with some of the top hospitals, such as Artemis Gurgaon and Teerthanker
Dr. Vivek Gupta is an experienced Surgical Oncologist with over 16 years of practice....
Senior Consultant
Medical Oncologist
Nanavati-Max Super Speciality Hospital, Mumbai
Book an Appointment Talk To ExpertSenior Director
Gynecologist and Obstetrician, IVF Specialist
Max Super Speciality Hospital, Shalimar Bagh, New Delhi
Book an Appointment Talk To ExpertSenior Director
Gynecologist and Obstetrician, IVF Specialist
Max Smart Super Speciality Hospital, Saket, New Delhi
Book an Appointment Talk To ExpertSenior Director
Gynecologist and Obstetrician
Max Smart Super Speciality Hospital, Saket, New Delhi
Book an Appointment Talk To ExpertSenior Director
Gynecologist and Obstetrician
Max Smart Super Speciality Hospital, Saket, New Delhi
Book an Appointment Talk To ExpertSenior Director
Gynecologist and Obstetrician
Max Smart Super Speciality Hospital, Saket, New Delhi
Book an Appointment Talk To ExpertFill up the form and get assured assitance within 24 hrs!
The Art of Effective Communication
 05 November,2024
Read More
05 November,2024
Read More
 29 October,2024
Read More
29 October,2024
Read More
 28 October,2024
Read More
28 October,2024
Read More
Trusted by Patients
"I am Asim from Bangladesh and was looking for treatment in India for neuro. I visited many websites to get the complete information regarding the treatment but I was not satisfied as I was getting confused. In the meanwhile, one of my friends suggested I seek help from Medi Journey as he experienced his medical journey very smoothly and was satisfied with it. They have filtered the top 10 doctors as per experience, the success rate of surgery & profile, so it helps us to choose the best treatment in India. "
"For my knee surgery, Medi Journey guided me to BLK Hospital where I received exceptional care. The team's support and the expertise at BLK Hospital exceeded my expectations. Thank you Medi Journey for making my medical journey stress-free. "
"I came from Iraq for my granddaughter's eye surgery in India facilitated by Medi Journey, due to critical cases they advised us to get a second opinion from the different hospitals before going to surgery. Finally, we went to Fortis Escort Hospital, which helped us to get more confidence for diagnosis. Fortis Escort Hospital has the best eye surgeon team with the latest instruments. Thanks to all team members for providing a high-quality treatment in India at an affordable cost. "
"I came for my hair transplant in India, before coming I was so confused about choosing the best clinic and surgeon for me. But thanks to God one of my friends had a hair transplant in India through Medi Journey. He recommended me to go with them. I am completely happy with my experience with them. They were always very fast in their responses to me. the success rate of my hair transplant surgery is 100%."
"Artemis Hospital, suggested by Medi Journey, turned out to be a great choice for my treatment. The personalized assistance and medical care were exceptional. I'm grateful to Medi Journey for guiding me to a hospital that perfectly matched my needs. Highly recommended! "
"I came from Afghanistan for my treatment in India at Jaypee Hospital, Noida. I had a fantastic experience with Medi Journey. Kudos to them for their incredible support during my medical journey. They not only took care of all the logistics but also connected me with a fantastic healthcare team. Efficient, caring, and highly recommended for a hassle-free medical tourism experience."
"I am Adam from Kano, Nigeria, one of my friends from Nigeria was facilitated by Medi Journey, and he recommended us to go with them. I sent my all reports to them and within 48 hours they reverted with 4 options from different hospitals. They helped me to get a Visa letter from the hospital, arrange pick-up from the airport, and book a hotel for me. Their team is very honest and throughout our stay in India they are with us they are caring for us like his family members. BLK Hospital is the best hospital in India with a top surgical oncologist surgeon team, a very advanced OT, and a Radiotherapy department. I wish more success to Medi Journey. "
"Great experience at the Max Hospital for my spine surgery and was successfully done. I thank my neurosurgeon and his entire team. I recommended all of my country's people to Medi Journey for treatment in India, they choose the best hospital, the best doctors, and the best cost for patients."
"I came to India from Dhaka, Bangladesh for my father-in-law's cardiac surgery at Fortis Hospital. I was confused about choosing the best surgeon for him before coming, but their team helped me to choose the best hospital and best cardiac surgeon in India with very good cost and 100% success rate of surgery. I am very happy with the services, really they make my journey so comfortable that make me feel at home. Thanks again and I like people to choose "Medi Journey" as your travel guide. "
"I am Mohammad from Bangladesh came to India for my general health checkup. Medi Journey offers me the complete package including Pick-up from the airport, hotel services, and 24-hour assistance. They guide you to choose the best hospital in India, the best cost of treatment with top-most doctors and give you complete information about hotel booking, and pick-up from the airport before coming to India They have the best team to help. Always choose Medi Journey for your treatment in India."





